Lecture Slides • Fall 2019
Main topics include:
• Syllabus
• succeeding in orgo I: best practices and reducing stereotype threat
• i>clicker, website
• define organic chemistry
• orbitals (Schrodinger equation)
• valence electrons
• models of bonding
• bond-line formulas
• Syllabus
• succeeding in orgo I: best practices and reducing stereotype threat
• i>clicker, website
• define organic chemistry
• orbitals (Schrodinger equation)
• valence electrons
• models of bonding
• bond-line formulas

The following slides were presented during class on the dates indicated. These slides are only made available after the indicated class date. Every effort is made to correct errors found on slides during lecture before posting.
Prelab slides can always be found at the end of the class lecture slides.
Prelab slides can always be found at the end of the class lecture slides.
"
Main topics include:
• review: 3 models of bonding
• molecular orbital (MO) theory
• valence electrons and formal charge
• drawing bond-line structural formulas
• resonance structures
• curved arrow notation
• determining major resonance contributors
• review: 3 models of bonding
• molecular orbital (MO) theory
• valence electrons and formal charge
• drawing bond-line structural formulas
• resonance structures
• curved arrow notation
• determining major resonance contributors
Main topics include:
• Arrenhius, Bronsted and Lewis acids
• conjugate pairs
• pKa scale
• structural effects on acidity: electronegativity, bond strength, resonance
• resonance contributors effect
• determining Keq from pKas
• Arrenhius, Bronsted and Lewis acids
• conjugate pairs
• pKa scale
• structural effects on acidity: electronegativity, bond strength, resonance
• resonance contributors effect
• determining Keq from pKas
Main topics include:
• Classes of hydrocarbons
• Hybridization (sp3)
• Bonding (VB) in hydrocarbons
• IUPAC nomenclature of alkanes
• Physical properties of alkanes: bp
• Van der Waals forces, London dispersion
• Polarizability
• Chemical properties: combustion
• Heat of combustion; • Solubility
• Classes of hydrocarbons
• Hybridization (sp3)
• Bonding (VB) in hydrocarbons
• IUPAC nomenclature of alkanes
• Physical properties of alkanes: bp
• Van der Waals forces, London dispersion
• Polarizability
• Chemical properties: combustion
• Heat of combustion; • Solubility
Main topics include:
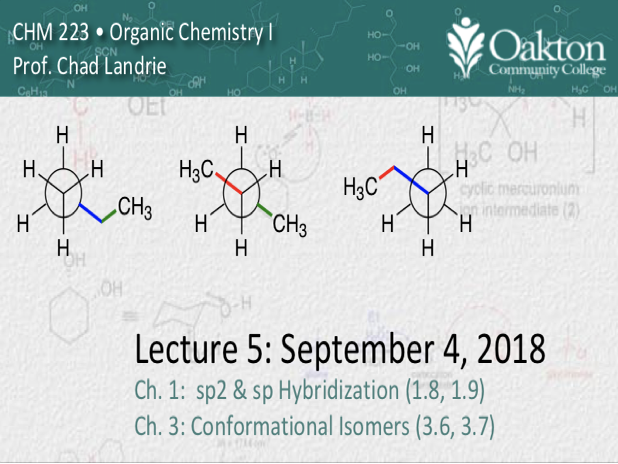
• sp2 and sp hybridization
• sigma bonds, pi bonds
• conformational isomers (tree)
• Newman, dash-wedge, sawhorse proj.
• eclipsed vs. staggered conformations
• eclipsed, gauche, anti relationships
• sp2 and sp hybridization
• sigma bonds, pi bonds
• conformational isomers (tree)
• Newman, dash-wedge, sawhorse proj.
• eclipsed vs. staggered conformations
• eclipsed, gauche, anti relationships
Main topics include:
• steric argument for conformer energies
• hyperconjugation argument
• conformational analysis of butane, sterics
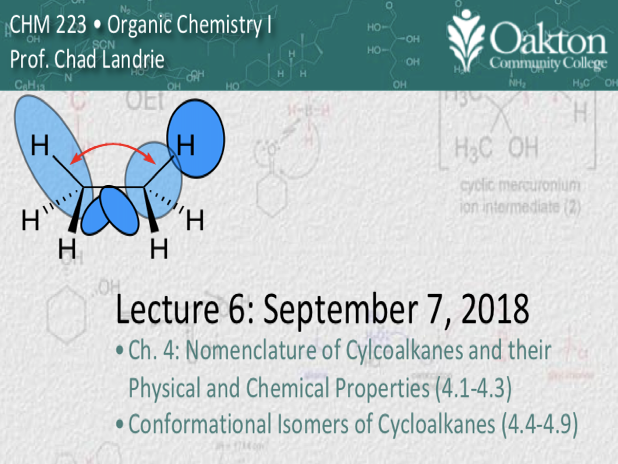
• cycloalkanes: naming, angle strain
• conformations of cycloalkanes
• steric argument for conformer energies
• hyperconjugation argument
• conformational analysis of butane, sterics
• cycloalkanes: naming, angle strain
• conformations of cycloalkanes
Main topics include:
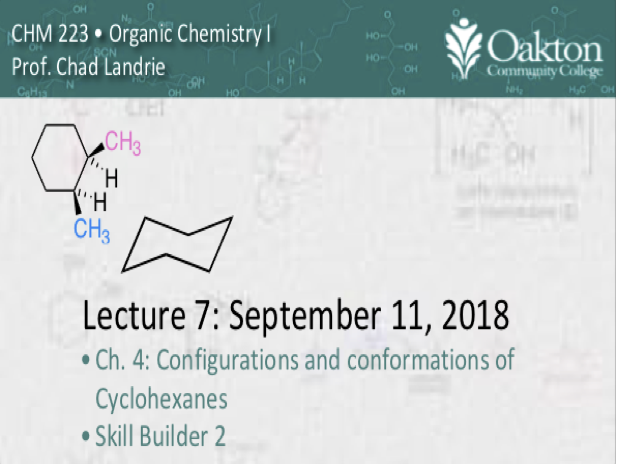
• conformations of cycloalkanes
• configurations of cycloalkanes
• 1,3-diaxial interactions
• chair and boat cyclohexanes, drawing
• cyclohexane ring inversion
• cis and trans configurational isomers
• Skillbuilder 2
• conformations of cycloalkanes
• configurations of cycloalkanes
• 1,3-diaxial interactions
• chair and boat cyclohexanes, drawing
• cyclohexane ring inversion
• cis and trans configurational isomers
• Skillbuilder 2
Main topics include:
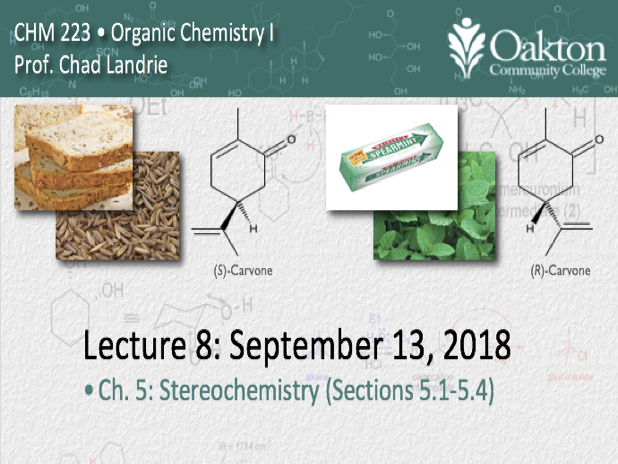
• chirality
• symmetry tests for chirality
• chirality centers, asymmetric carbon
• chirality centers of N, S and P
• pyramidal inversion
• stereogenic centers
• enantiomers
• CIP sequence rules (R/S-notation)
• chirality
• symmetry tests for chirality
• chirality centers, asymmetric carbon
• chirality centers of N, S and P
• pyramidal inversion
• stereogenic centers
• enantiomers
• CIP sequence rules (R/S-notation)
Main topics include:
• Fisher projections
• optical rotation
• demonstration of homemade polarimeter
• enantiomeric excess (ee) and [a]
• diastereomers and properties
• mess forms
• determining total number of stereoiosmers
• Fisher projections
• optical rotation
• demonstration of homemade polarimeter
• enantiomeric excess (ee) and [a]
• diastereomers and properties
• mess forms
• determining total number of stereoiosmers
Main topics include:
• IR of functional groups
• EWG and EDG effect of IR of carbonyls
• nuclear magnetic resonance, theory
• precession; Larmor frequency
• Faraday induction
• free induction decay (FID)
• chemical shift scale, TMS
• shielding effect
• IR of functional groups
• EWG and EDG effect of IR of carbonyls
• nuclear magnetic resonance, theory
• precession; Larmor frequency
• Faraday induction
• free induction decay (FID)
• chemical shift scale, TMS
• shielding effect
Main topics include:
• structural effects: EN, #Hs, anisotropy
• examples of NMR spectra and structural effect on chemical shift value
• H-NMR equivalency and # signals
• homotopic, enantiotopic, diastereotopic, heterotopic H-atoms
• test for equivalency
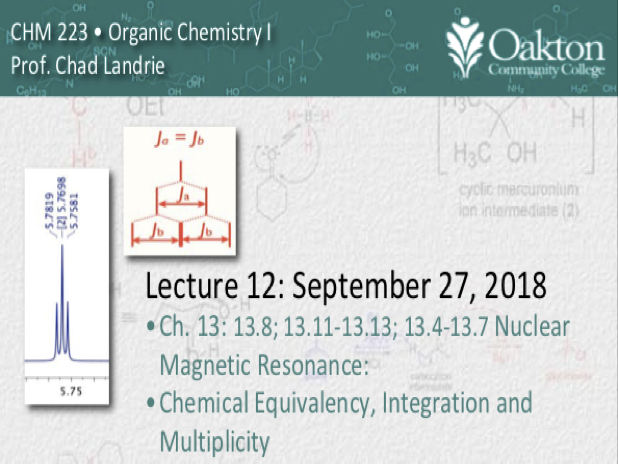
• multiplicity, spin-spin splitting
• n+1 rule
• structural effects: EN, #Hs, anisotropy
• examples of NMR spectra and structural effect on chemical shift value
• H-NMR equivalency and # signals
• homotopic, enantiotopic, diastereotopic, heterotopic H-atoms
• test for equivalency
• multiplicity, spin-spin splitting
• n+1 rule
Main topics include:
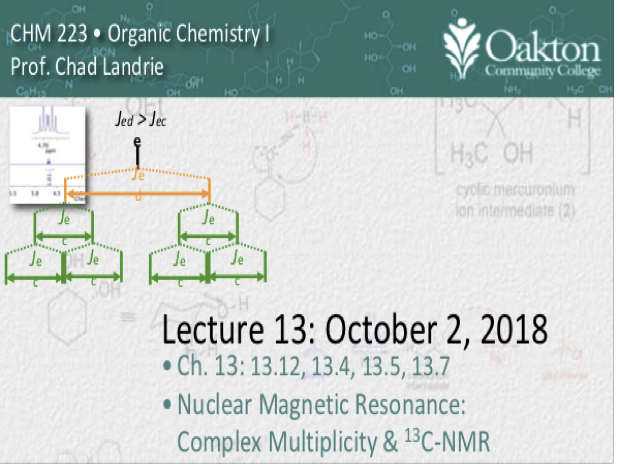
• complex multiplicities
• splitting trees
• coupling constant (J-value)
• peak distortion (leaning)
• 13C-NMR
• DEPT
• Practice
• complex multiplicities
• splitting trees
• coupling constant (J-value)
• peak distortion (leaning)
• 13C-NMR
• DEPT
• Practice
Main topics include:
• intro to alcohols and alkyl halides
• nomenclatures of alcohols and halides
• physical properties: polarity, bp
• intro to chemical reactions
• bond dissociation energies (BDE)
• calculating enthalpy of reaction
• reaction coordinate diagrams, Ea
• intermediates and transition states
• intro to alcohols and alkyl halides
• nomenclatures of alcohols and halides
• physical properties: polarity, bp
• intro to chemical reactions
• bond dissociation energies (BDE)
• calculating enthalpy of reaction
• reaction coordinate diagrams, Ea
• intermediates and transition states
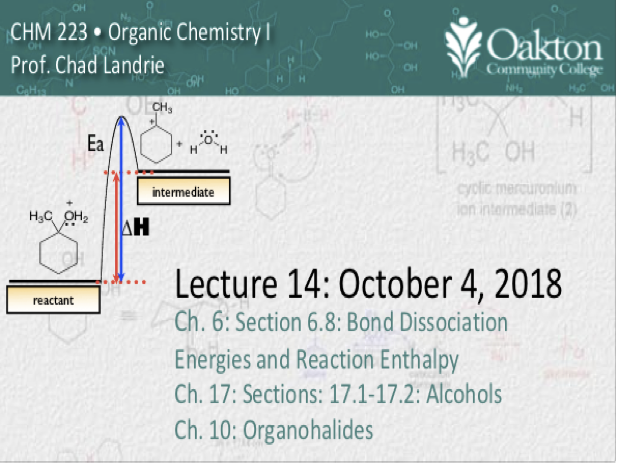
Main topics include:
• reactivity of alcohols
• reactivity of hydrogen halides
• SN1 mechanism, carbocations
• carbocation stability: inductive effect and hyperconjuation
• Hammond postulate
• transition state energies
• SN2 mechanism and rate laws
• reactivity of alcohols
• reactivity of hydrogen halides
• SN1 mechanism, carbocations
• carbocation stability: inductive effect and hyperconjuation
• Hammond postulate
• transition state energies
• SN2 mechanism and rate laws
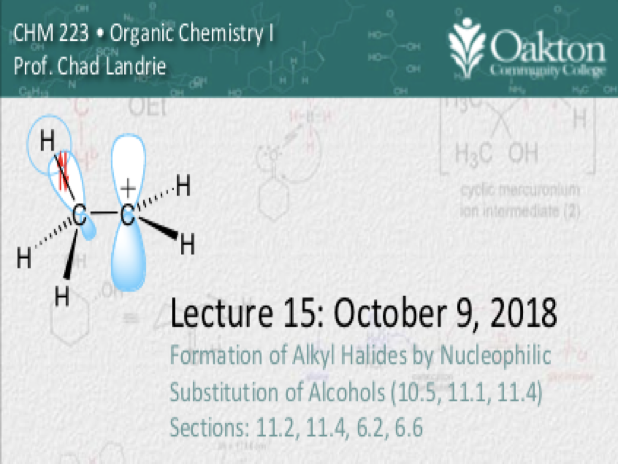
Main topics include:
• nucleophiles and electrophiles
• definition and examples
• examples of SN2 with various nucs.
• reactivity of halide leaving groups
• stereochemistry of SN2; stereospecific
• steric effects on the rate os SN2
• strength of nucleophiles; nucleophilicity
• nucleophiles and electrophiles
• definition and examples
• examples of SN2 with various nucs.
• reactivity of halide leaving groups
• stereochemistry of SN2; stereospecific
• steric effects on the rate os SN2
• strength of nucleophiles; nucleophilicity
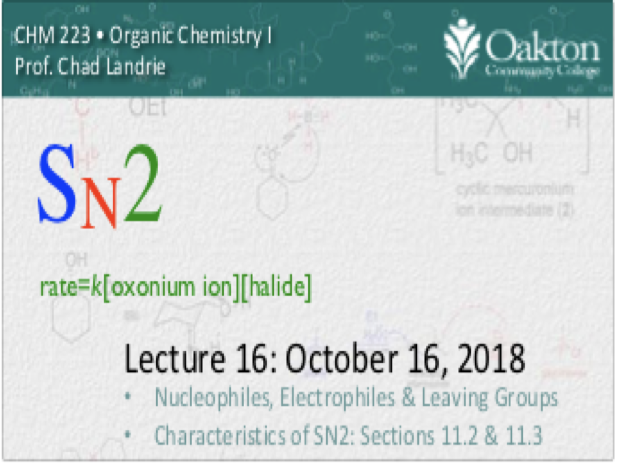
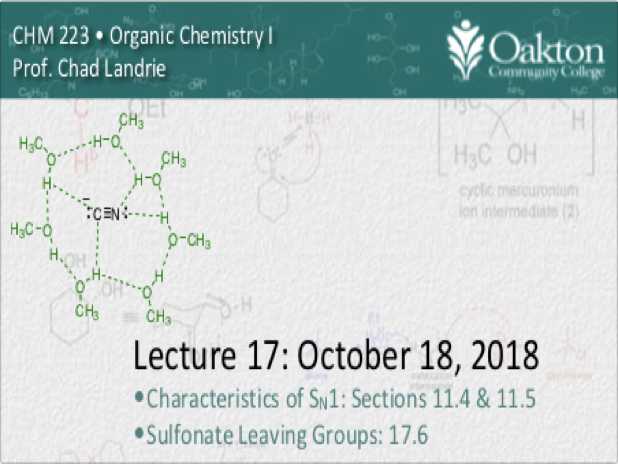
Main topics include:
• review SN1 and SN2 mechanism
• solvolysis; SN1 and SN2 with neutral nut
• carbocation stabiliti: resonance effect
• carbocation stability: solvent effect on the rate of SN1
• polar aprotic solvents: rate effect on SN2
• stereochemistry of SN1: ion pairs
• sulfonate leaving groups; preparation
• review SN1 and SN2 mechanism
• solvolysis; SN1 and SN2 with neutral nut
• carbocation stabiliti: resonance effect
• carbocation stability: solvent effect on the rate of SN1
• polar aprotic solvents: rate effect on SN2
• stereochemistry of SN1: ion pairs
• sulfonate leaving groups; preparation
Main topics include:
• review alkene structure and hybridize
• review IHD
• E/Z nomenclature; naming alkenols
• CIP priority review
• stability of alkenes; heat of combustion
• cis vs trans stability
• intro to B-eliminations
• review alkene structure and hybridize
• review IHD
• E/Z nomenclature; naming alkenols
• CIP priority review
• stability of alkenes; heat of combustion
• cis vs trans stability
• intro to B-eliminations
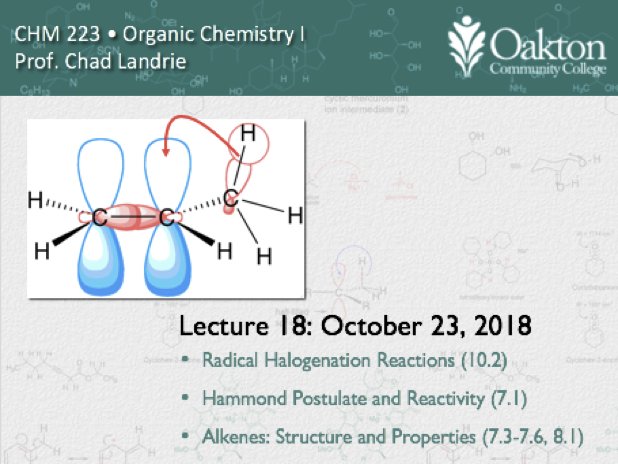
Main topics include:
• mechanism of E1 & E2 dehydration
• carbocation rearrangements
• E2 dehydrohalogenation
• reactivity and mechanism
• anti elimin. for dehydrohalogenation: torsional strain and hyperconjugation
• stereospecific E2
• mechanism of E1 & E2 dehydration
• carbocation rearrangements
• E2 dehydrohalogenation
• reactivity and mechanism
• anti elimin. for dehydrohalogenation: torsional strain and hyperconjugation
• stereospecific E2
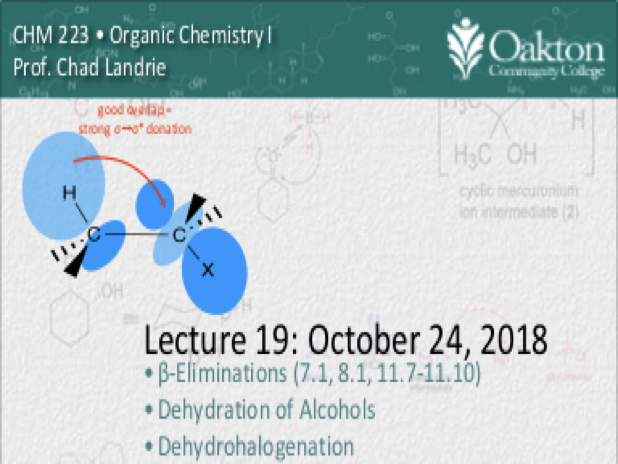
Main topics include:
• Competition between substitution and elimination; conditions
• addition reactions to alkenes
• hydrogenation; stereoselectivity
• Markovnikov addition of HX
• peroxide effect; anti-Markovnikov
• addition of H2SO4 to alkenes
• hydration of alkenes
• Competition between substitution and elimination; conditions
• addition reactions to alkenes
• hydrogenation; stereoselectivity
• Markovnikov addition of HX
• peroxide effect; anti-Markovnikov
• addition of H2SO4 to alkenes
• hydration of alkenes

Main topics include:
• Hydroboration-Oxidation
• Halogenation; vicinal halohydrins
• Rates of addition to alkenes
• ozonolysis
• dihydroxylation
• epoxidation
• synthesis; retrosynthetic analysis
• Hydroboration-Oxidation
• Halogenation; vicinal halohydrins
• Rates of addition to alkenes
• ozonolysis
• dihydroxylation
• epoxidation
• synthesis; retrosynthetic analysis
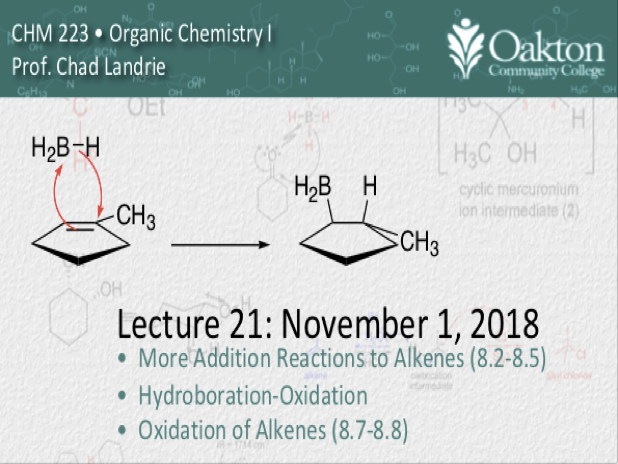
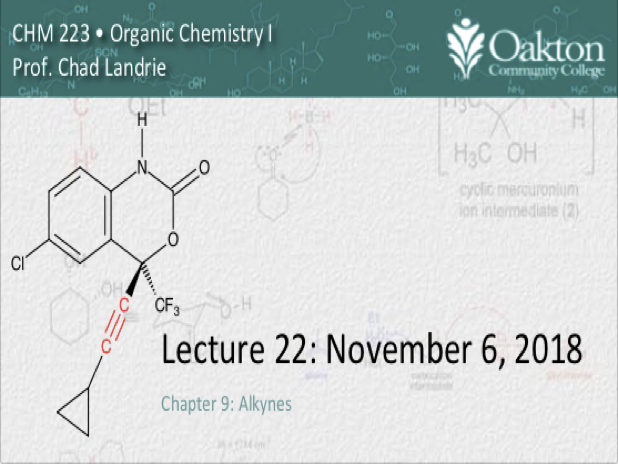
Main topics include:
• Alkynes: physical and chemical props.
• alkyne acidity; common bases
• alkylation of alkynes with alkyl halides
• double dehydrohalogenation
• addition reactions of alkynes: HX, hydration by Hg2+, halogenation,
• hydrogenation; Lindlar catalyst (cis)
• dissolving metal reduction (trans)
• Skillbuilder 4
• Alkynes: physical and chemical props.
• alkyne acidity; common bases
• alkylation of alkynes with alkyl halides
• double dehydrohalogenation
• addition reactions of alkynes: HX, hydration by Hg2+, halogenation,
• hydrogenation; Lindlar catalyst (cis)
• dissolving metal reduction (trans)
• Skillbuilder 4
Main topics include:
• reactions of alkynes: Hg cat. hydration, halogenation, HX addition, hydroboration
• conjugated systems; allylic carbocations
• SN1 and SN2 reactivity of allylic halides
• allylic radical halogenation
• NBS and NCS
• reactions of alkynes: Hg cat. hydration, halogenation, HX addition, hydroboration
• conjugated systems; allylic carbocations
• SN1 and SN2 reactivity of allylic halides
• allylic radical halogenation
• NBS and NCS
Main topics include:
• review of allylic substitution runs
• classes and nomenclature of dienes
• conformations of conjugated dienes
• E1 and E2 of allylic and homoallylic halides gives conjugated dienes
• kinetic vs thermodynamic addition
• Diels-Alder Reaction; stereospecificity
• Diels-Alder activating groups
• review of allylic substitution runs
• classes and nomenclature of dienes
• conformations of conjugated dienes
• E1 and E2 of allylic and homoallylic halides gives conjugated dienes
• kinetic vs thermodynamic addition
• Diels-Alder Reaction; stereospecificity
• Diels-Alder activating groups
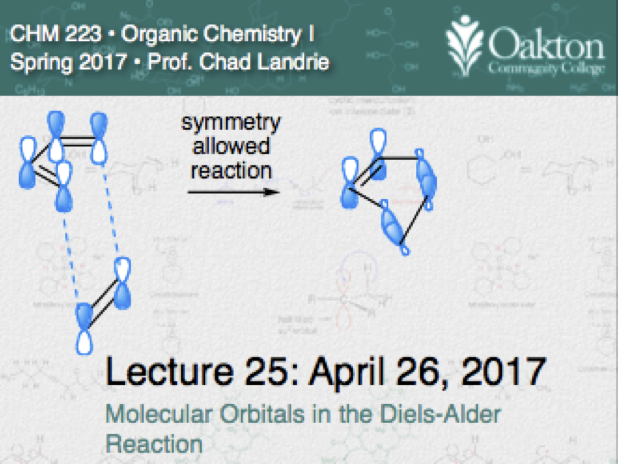
Main topics include:
• Frontier molecular orbitals (FMOs)
• Constructing the HOMO and LUMO of conjugated systems
• predicting symmetry allowed cycloadditions
• Frontier molecular orbitals (FMOs)
• Constructing the HOMO and LUMO of conjugated systems
• predicting symmetry allowed cycloadditions
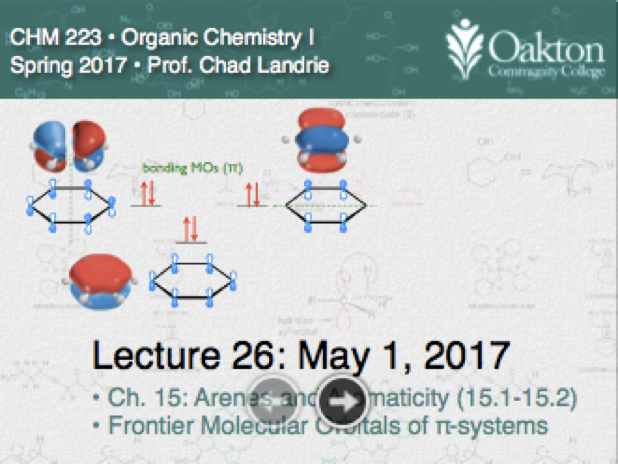
Main topics include:
• Benzene: observations of aromaticity
• Constructing MOs of benzene
• Defining aromaticity as closed shell for cyclically conjugated systems
• Frost Circle; Antiaromatic systems
• Consequences of aromaticity: tropylium
• Benzene: observations of aromaticity
• Constructing MOs of benzene
• Defining aromaticity as closed shell for cyclically conjugated systems
• Frost Circle; Antiaromatic systems
• Consequences of aromaticity: tropylium
Main topics include:
• Reactions of benzylic carbons: styrenyl additions; halogenation, SN1, SN2, E1, E2
• Clemensen and Wolf-Kishner reduction
• Hydrogenation of benzylic ketones
• KMnO4 oxidation of benzylic carbons
• Electrophilic aromatic substitution
• nitration, sulfonation, bromination
• Friedel-Crafts alkylation and acylation
• Reactions of benzylic carbons: styrenyl additions; halogenation, SN1, SN2, E1, E2
• Clemensen and Wolf-Kishner reduction
• Hydrogenation of benzylic ketones
• KMnO4 oxidation of benzylic carbons
• Electrophilic aromatic substitution
• nitration, sulfonation, bromination
• Friedel-Crafts alkylation and acylation
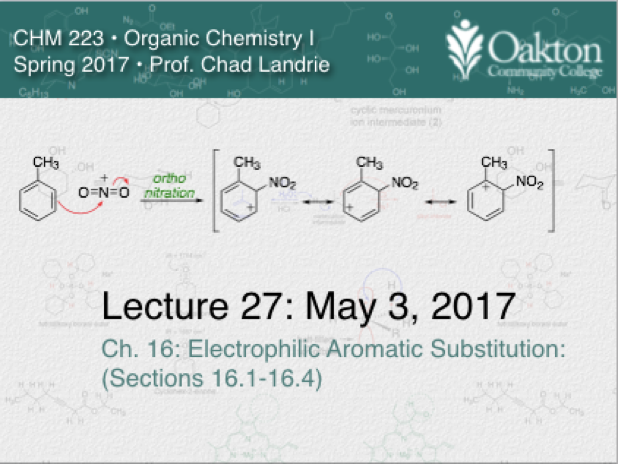
Main topics include:
• Electrophilic aromatic substitution
• ortho/para directors
• meta directors
• explanation for regioselectivity
• multiple directing effects
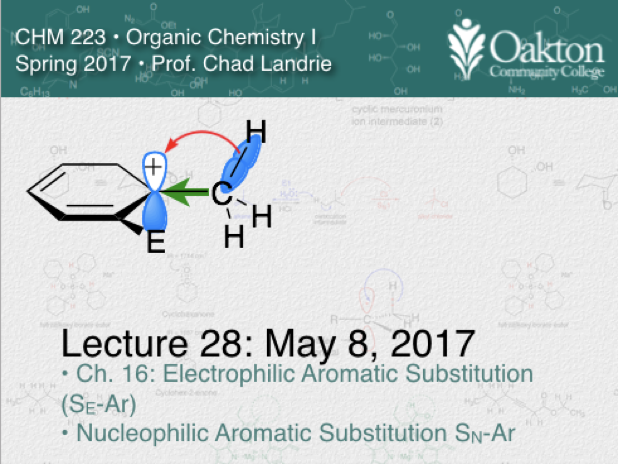
• nucleophilic aromatic substitution
• addition-elimination mechanism
• Electrophilic aromatic substitution
• ortho/para directors
• meta directors
• explanation for regioselectivity
• multiple directing effects
• nucleophilic aromatic substitution
• addition-elimination mechanism
Main topics include:
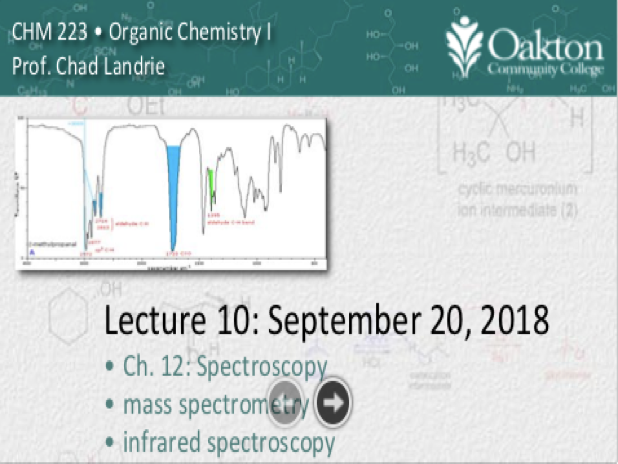
• spectroscopy vs. spectrometry
• mass spectrometry, spectrometer
• Lorentz forces, right-hand rule
• MS spectrum: base peak, M+ and M+1
• infrared spectroscopy, electromagnetic
• stretching, bending movements, dipoles
• wave numbers, areas of IR spectrum
• examples of IR functional groups
• spectroscopy vs. spectrometry
• mass spectrometry, spectrometer
• Lorentz forces, right-hand rule
• MS spectrum: base peak, M+ and M+1
• infrared spectroscopy, electromagnetic
• stretching, bending movements, dipoles
• wave numbers, areas of IR spectrum
• examples of IR functional groups